Abstract
Objective: To inform regulatory decision making about trial design and drug approval, we examined whether disease response status is a patient-centered endpoint in therapeutic trials for acute myeloid leukemia (AML). Specifically, we estimated the association of response status with patient-reported symptoms and physical function in the Beat-AML Master Trial.
Methods: The Beat-AML Master Trial (NCT03013998) is composed of a genomic screening protocol and multiple sub-studies of biomarker-based treatments for sub-types of acute myeloid leukemia. The patient-reported outcome (PRO) assessment occurred at baseline and at specified study visits about once per cycle depending on treatment phase. The PRO assessment included the MD Anderson Symptom Inventory (MDASI) AML/MDS symptom scale (17 items), additional symptom items from the MDASI library relevant to Beat-AML therapies (10 items), and the PROMIS® Physical Function short form (6 items). The two symptom scale scores range from 0-10 where higher scores indicate greater symptom burden; the physical function scale score ranges from 0-100 where higher scores indicate better physical function. Disease response was assessed according to 2017 ELN AML Recommendations, including: complete remission (CR), complete remission without minimal residual disease (CRMRD-), complete remission with hematologic improvement (CRh), complete remission with incomplete blood count recovery (CRi), morphological leukemia free state (MLFS), partial remission (PR), stable disease, progressive disease, recurrence/relapse, and treatment failure. For this analysis, response status was identified for each PRO assessment timepoint. Linear mixed effects models estimated the differences in PRO scores between each response status and CR. The analytic sample was composed of individuals who completed the baseline PRO assessment and at least one PRO assessment post baseline. The minimally important difference (MID) for the PROMIS Physical Function scale is 4 points (Yost, J Clin Epi 2012). The symptom scales do not have established MID, so coefficients were compared to a small effect size (0.20) per Cohen's d.
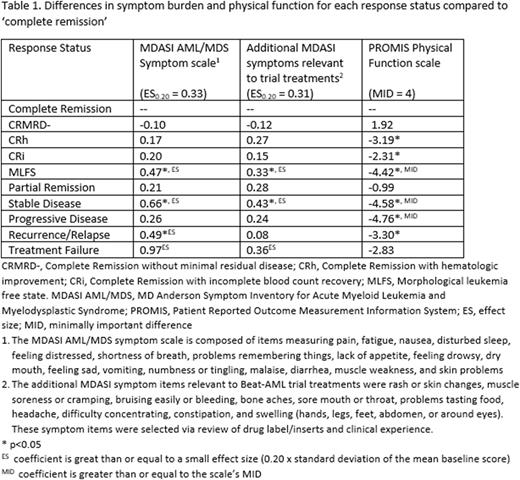
Results: The analytic sample included 240 patients from 11 Beat-AML sub-studies; the median age was 73 years (range: 42-90) and 42.5% were female. Median follow-up time with PRO assessments was 18.4 months. The survey response rate was 80.7%. The mean (standard deviation) PRO scale scores at baseline were: 2.11 (1.67) AML/MDS symptom scale, 1.54(1.54) additional symptoms, and 36.65(9.62) Physical Function. The proportion of patients achieving each response at any point during protocol treatment was 32.1% CR, 7.5% CRMRD-, 6.7% CRh, 23.3% CRi, 19.6% MLFS, 5.8% PR, 73.3% stable disease, 11.7% progressive disease, 20.0% recurrence/relapse, and 7.5% treatment failure, for an average of 2.4 responses per patient. After controlling for age, gender, treatment (sub-study), time since baseline, and adjusting for drop-out missingness, we found that, compared to CR, symptom burden was greater for MLFS and stable disease (coefficient range 0.33 to 0.66) (see Table 1). Symptom burden per the AML/MDS scale only was also greater for recurrence/relapse (coef. 0.49). Physical function was worse (per MID) for MLFS, stable disease, and progressive disease (coefficient range: -4.42 to -4.76).
Discussion: PRO data from 11 sub-studies of the Beat AML Master Trial indicate key differences and similarities between disease response statuses in terms of symptom burden and physical function. MLFS was the only type of response that differed from CR with respect to symptom burden and physical function. Stable disease and recurrence/relapse were associated with greater symptom burden than CR, but progressive disease was not, however it was associated with worse physical function. Findings regarding treatment failure may be limited by small sample and wide range of health within that status. Sensitivity analyses will include removing rare symptoms from the symptom scales, which may increase model coefficients. Subsequent analysis will examine the association of the patient's best response status with long term physical function.
Conclusion: Further research is warranted to establish the response statuses of CRMRD-, CRh, CRi, and partial remission as clinical trial endpoints that are valuable to patients with respect to health-related quality of life.
Disclosures
Foster:Daiichi Sankyo: Consultancy; Macrogenics: Consultancy, Research Funding; Bellicum Pharmaceuticals: Research Funding. Bryant:Carevive Systems, Inc: Research Funding. Wood:Pfizer: Research Funding; Genentech: Research Funding; Teladoc: Consultancy. Mims:Daiichi Sankyo: Other: Data Safety and Monitoring Board; Astellas: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Other: Data Safety and Monitoring Board; Syndax: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Zentalis: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Ryvu: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Borate:Novartis: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie/Genentech: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal